
Research
Group of Plant-Insect Interactions
 |
Link to group homepage |
Faculty staff
 |
Prof. Dr. Ivan GALIS E-mail:igalisATokayama-u.ac.jp |
(please change AT to @ before sending) | ||
 |
Assoc. Prof. Dr. Tomonori SHINYA E-mail:shinyatATrib.okayama-u.ac.jp |
(please change AT to @ before sending) |
Lectures: Plant Genetics and Biotic Stress Science, Topics in Plant-Insect Interactions
Keywords: Plant defense mechanisms; Direct and indirect defense; Insect herbivores
Summary of main research topics
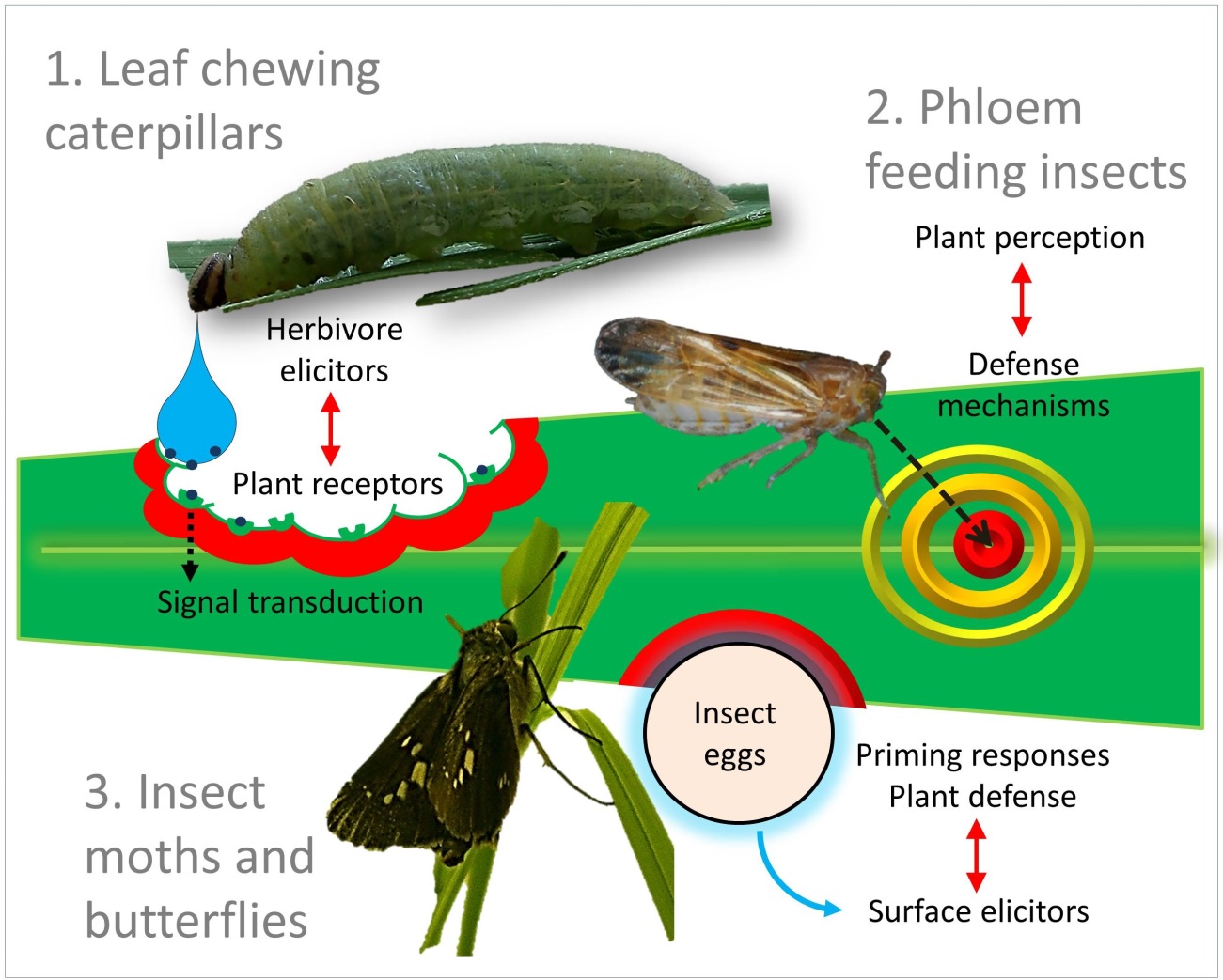
| Elucidation of plant-insect interactions at the molecular level Establishment of effective plant defense systems against herbivores in natural history reflects the existence of extremely variable interactions between plants and insects, also known as co-evolution process. Our group strives to understand, at a molecular level, the mechanisms of activation, signal transduction and metabolic basics of plant defenses triggered after the recognition of insect attack. Furthermore, we target sustainable pest control by the use of natural enemies and their attraction to herbivore-infested plants by the emissions of various volatile organic compounds (VOCs) from plants. |
 |
Latest publications (for complete and most current publications visit group pages)
(1) Osibe D.A, Hojo Y, Shinya T, Mitani-Ueno N, Galis I. Comprehensive analysis of silicon impact on defense and metabolic responses in rice exposed to herbivory stress. Front. Plant Sci. 15: 1399562. doi.org/10.3389/fpls.2024.1399562 (2024. 5.)
(2) Osibe D.A. Study on the role of mineral elements in rice defense against herbivores. 博士学位論文(岡山大学)(2024. 9.)
(3) Yamaji N, Mitani-Ueno N, Fujii T, Shinya T, Shao J.F, Watanuki S, Saitoh Y, Ma J.F. Shoot-Silicon-Signal protein to regulate root silicon uptake in rice. Nature Communications 15: 10712. doi.org/10.1038/s41467-024-55322-7 (2024. 12.)
(4) Kanda Y, Shinya T, Wari D, Hojo Y, Fujiwara Y, Tsuchiya W, Fujimoto Z, Thomma B.P.H.J, Nishizawa Y, Kamakura T, Galis I, Mori M. Chitin-signaling dependent responses to insect oral secretions in rice cells propose the involvement
of chitooligosaccharides in plant defense against herbivores. Plant J. 121: e17157. doi.org/10.1111/tpj.17157 (2024.11. Online preview.)





